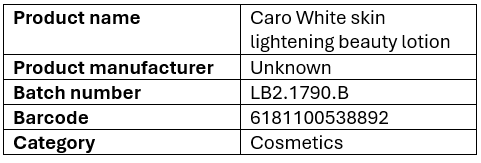
The European Union (EU) Rapid Alert System for Dangerous Non-Food Products (RAPEX) recently recalled Caro White Skin Lightening Beauty Lotion, and the National Agency for Food and Drug Administration and Control, NAFDAC, has issued a public notification.
Caro White was recalled because it did not comply with the EU Cosmetic Products Regulation, which mandates a maximum of 1% kojic acid, as concluded by the EU Scientific Committee on Consumer Safety (SCCS).
Kojic acid plays a significant role in skin-lightening beauty lotions because it inhibits melanin production, the pigment that gives skin its colour.
The high concentration of Kojic Acid raises concerns about its potential endocrine-disrupting properties.
NAFDAC advises importers, distributors, retailers, and consumers to be cautious and vigilant regarding the product. Although the product is not listed in its database, NAFDAC advises careful authentication of the product and an assessment of its physical condition.
The public is advised to report any inexperience through the use of the product to the nearest NAFDAC office or send an email via pharmacovigilance@nafdac.gov.ng.
Source: Nairametrics